Last Updated on July 3, 2022
In most refrigeration and air-conditioning systems used throughout the world vapor compression cycle is used with a refrigerant as working fluid. The vapor compression cycle though demonstrated usually is theoretical and not the actual one.
Actual vapor compression cycle is different from theoretical vapor compression cycle. In actual vapor compression cycle condensation and evaporation do not occur at constant pressure. Further compression is not isentropic and the overall COP of the cycle is lower.
The actual vapor compression cycle is thereby less effective as compared to theoretical vapor compression cycle. Additionally one of the major factors playing the role in less cop is friction during the route of working fluid or refrigerant.
All air conditioners and refrigerators use actual vapor compression cycle. The cycle can be brought closer to theoretical vapor compression cycle but it can never be made to overlap theoretical vapor compression cycle.
No doubt actual vapor compression cycle reduces COP and effectiveness of the cooling process. However in some ways it is favorable as it allows sub cooling in condenser and superheating in compressor. This enables prevention of liquid droplet in the compressor that can damage it.
What is vapor compression cycle?
Vapor compression cycle allows the extraction of heat from low temperature zone and transfers it to high temperature zone. It is most common cycle used in refrigerators.
Vapor compression cycle consists of 4 basic processes:
- Compression
- Condensation
- Throttling
- Evaporation.
A brief working of the processes included in vapor compression cycle are provided below:
Compression
Initially the refrigerant is compressed adiabatically in the compressor. This allows the low temperature and low pressure refrigerant to be converted to high pressure and high temperature refrigerant.
Electrically operated reciprocating compressor are used usually. However compressors are of several types including centrifugal, screw, scroll etc.
The volume decrease with the increase in pressure. This indicates increment in the internal energy due to decrease in the spacing in between refrigerant molecules.
Condensation
After compression high pressure and high temperature fluid is allowed to move into condenser. Condenser plays the role of removal of heat from the refrigerant at high pressure.
The heat removed from the refrigerant theoretically neither affects pressure nor temperature. Thereby this process is isobaric (constant pressure) as well as isothermal (constant temperature)
The enormous amount of heat removed from the refrigerant in the condenser is called heat of condensation.
- Air-cooled
- Water cooled
Throttling and expansion
Throttling or expansion process allows decrease in pressure and temperature of the refrigeration after passing from throttling valve.
The valve actually allows sudden increase in the cross-sectional area that expands the refrigerant decreasing its pressure. The refrigerant is almost 75% liquid after this stage.
Despite cooling of the refrigerant through expansion, throttling actually controls the amount of liquid sent to evaporator.
Evaporation
Evaporator is actually the output section of the refrigerator or air-conditioning system. It is the component that accounts for the refrigeration effect induced by the cooling system.
Evaporator allows the absorption of heat through the surrounding as the refrigerant moves through the refrigerant coils. The process is called evaporation.
Theoretically evaporation is isothermal as there is no change in the temperature of refrigerator as the refrigerant boils during its movement through the evaporation coils.
The heat absorbed by the application of evaporation is thereby called latent heat of vaporization (latent heat required to convert liquid refrigerant to vapor). It is the same as heat of condensation as the energy required to convert liquid refrigerant to vapor and vice versa is the same.
Normally there are two types of evaporators:
- Air-cooling
- Water-cooling
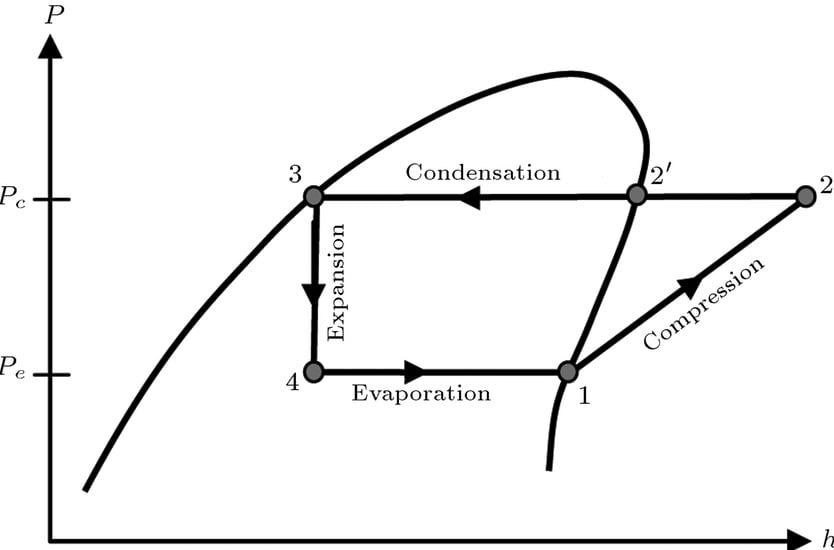
Theoretical or Ideal Vapor Compression Cycle
Theoretical vapor compression cycle is the same as discussed above. The major process on ph diagram given above are given below:
Isentropic Compression
Increment of pressure with reduction of volume with constant entropy.
Isobaric Condensation
Loss of heat with phase change at constant pressure (and constant temperature).
Isenthalpic expansion
Decrease in pressure and increase in volume with constant enthalpy.
Isobaric evaporation
Absorption of heat with phase change at constant pressure (and constant temperature)
Comparison of Actual and Theoretical Vapor Compression Cycle
The cycle that is used in all the cooling devices is in fact the actual vapor compression cycle. It has less COP and it requires more work. However at the same time some changes are intentional.
Major reasons of less efficiency of this cycle are mechanical and fluid friction and heat loss to the surroundings.
I have summarized processes in actual vapor compression cycle compared to theoretical vapor compression cycle below:
Compression
In an ideal vapor compression cycle refrigerant is compressed isentropically. This means no change in entropy during compresson.
And isentropic process also implies that the process is adiabatic involving no heat loss. This mean in an ideal vapor compression cycle the process of compression is reversible.
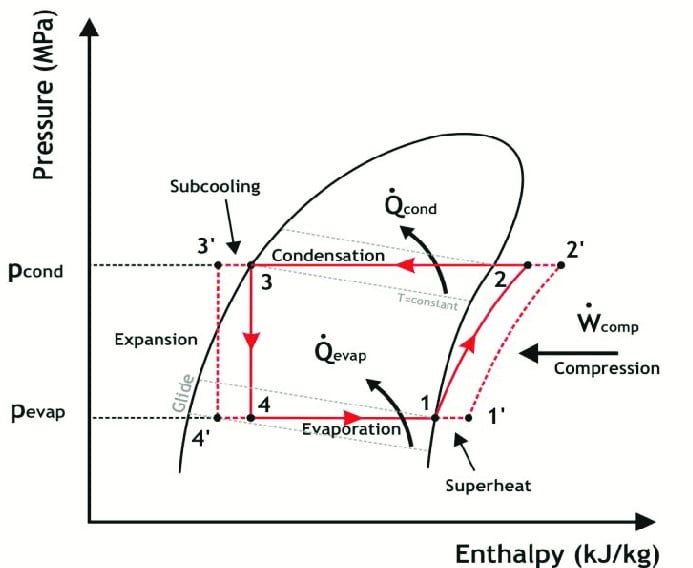
However in actual vapor compression cycle we have to include the other effects i.e. Fluid friction and mechanical friction. The process is showed in the diagram above by process 2 – 3.
Due to friction the compression process is no longer isentropic as the friction results in the formation of heat. The formation of heat results in increment of entropy.
Thereby the compression in actual vapor compression cycle is converted from reversible to irreversible process. This irreversibility is one the major reason of the decrement of cop in actual vapor compression cycle.
The compression will also heat the liquid refrigerant above the temperature that it did in theoretical vapor compression cycle. Hence the refrigerant will enter the condenser at higher temperature.
Condensation
It is represented in the figure by process 4 – 6. In actual vapor compression cycle refrigerant enters the condenser at higher temperature as compared to ideal vapor compression cycle. This is due higher work done during compression in actual vapor compression cycle by talking friction in account.
Ideally when heat is released during the process of condensation it occurs isobarically and isothermally. This implies no change in pressure as well as temperature during ideal vapor compression cycle.
However in actual this is not the case. The loss of heat is mainly though phase change however there is noticeable change in temperature during condensation.
Further the pressure is not constant in actual vapor compression cycle during the process as in ideal case. This is due to fact that slight expansion during the release of heat when the fluid is travelling through lines of the condenser is in evitable. The decrease in pressure is experienced due to this.
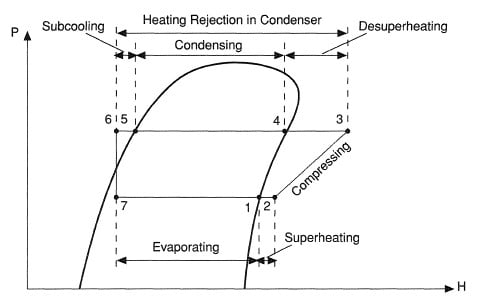
Contrary to ideal vapor compression cycle the refrigerant is not in the saturated liquid state at the end of condensation. In fact it is cooled beyond the saturated liquid. This is governed by a process known as subcooling.
Subcooling is the process cooling the refrigerant beyond the saturated liquid state such that it has lower enthalpy as compared to if liquid was in saturated liquid state. It is represented by process 5 – 6.
Subcooling is however an appreciable process as it ensure that all the refrigerant is converted into liquid state before it is expanded in the expansion or throttle valve.
Expansion
In the diagram it is indicated by the process represented by line 6-7.
The major difference between ideal and actual vapor compression cycle in throttling is the enthalpy. The fluid entering the throttling valve in actual vapor compression cycle has lower enthalpy than in ideal vapor compression cycle and saturated liquid state.
The process of subcooling yields comparatively lower heat content at the end of condensation as compared to actual vapor compression cycle.
The higher release of heat in the condensation during subcooling results in lower temperature achieved by expansion of refrigerant in actual vapor compression cycle as compared to ideal vapor compression cycle. This is due to the fact that the fluid entered in the throttling valve at lower heat content.
Evaporation
It is represented in the figure by process 6-7. In actual vapor compression cycle refrigerant enters the evaporator at lower temperature as compared to ideal vapor compression cycle. This is due subcooling in condensation that allowed fluid to drop more temperature after expansion.
Ideally when heat is absorbed during the process of evaporation it occurs isobarically and isothermally. This implies no change in pressure as well as temperature during ideal vapor compression cycle.
However in actual this is not the case. The absorption of heat is mainly though phase change however there is noticeable change in temperature during evaporation.
Further the pressure is not constant in actual vapor compression cycle during the process as in ideal case. This is due to fact that slight expansion during the absorption of heat when the fluid is travelling through lines of the evaporator is in evitable. The decrease in pressure is experienced due to this.
Contrary to ideal vapor compression cycle the refrigerant is not in the saturated vapor state at the end of evaporation. In fact it is heated beyond the saturated vapor state. This is governed by a process known as superheating.
Superheating is the process of heating the refrigerant beyond the saturated vapor state such that it has higher enthalpy as compared to if refrigerant was in saturated vapor state.
Superheating is considerably significant process as it ensure that all the refrigerant is converted into vapor state before it enters the compressor. This prevents possible damage to the compressor that may occur by partially liquid refrigerant.
Conclusion:
On comparison of ideal/theoretical and actual vapor compression cycle it is evident that the supposition in the ideal refrigeration cycle is to make calculations easier.
The perfectly reversible processes like isentropic compression and isothermal heat absorption and rejection can never be devised practically in a cycle.
However some processes like subcooling and superheating actually help in right operation of the components of refrigerator. Hence actual vapor compression cycle in this way is intentional and favorable.